- by foxnews
- 14 Mar 2025
US FDA considers approving second Covid-19 booster shot - report
US FDA considers approving second Covid-19 booster shot - report
- by theguardian
- 20 Feb 2022
- in news
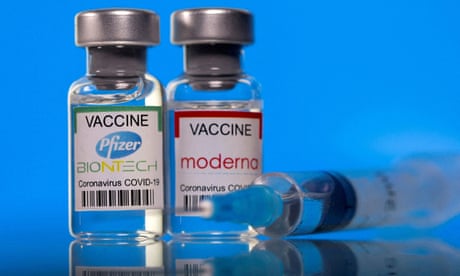
US health regulators are looking at authorizing a potential fourth dose of a Covid-19 vaccine in the fall, the Wall Street Journal reported on Saturday, citing sources familiar with the matter.
The Food and Drug Administration (FDA) has been reviewing data to authorize a second booster dose of vaccines from Pfizer and BioNTech and Moderna, the report added.
The FDA did not immediately comment.
The agency last month cut the interval to get a booster dose of Covid-19 vaccines from Pfizer and BioNTech as well as from Moderna, in a bid to provide better protection sooner against the Omicron variant.
The planning is still in early stages and authorization would depend on determinations as to whether the second booster should be authorized for all adults or particular age groups, and whether it should target the Omicron variant or be formulated differently, the report said.
It added that no decision was final and that it could be necessary to make booster shots available earlier if a new variant appears.
The US reported 2,323 Covid-19 deaths on Friday, bringing the overall death toll to 936,523.
- by foxnews
- descember 09, 2016
Southwest flyers fire back over airline ending free checked bag policy: 'Nail in the coffin'
Southwest has customers sounding off after the airline announced an end to its checked bag policy, leading some flyers to say they'll "boycott" the airline.
read more


