- by foxnews
- 07 Apr 2025
Moderna says booster produces strong antibody response to Omicron
Moderna says booster produces strong antibody response to Omicron
- by theguardian
- 21 Dec 2021
- in news
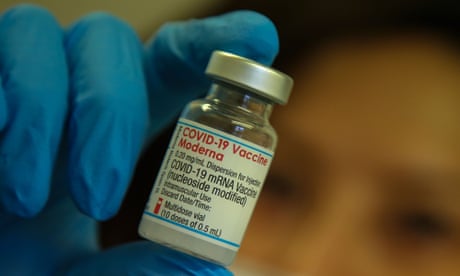
The decision to focus on the current vaccine, mRNA-1273, was driven in part by how quickly the variant is spreading. The company plans to develop a vaccine specifically to protect against Omicron, which it hopes to advance into clinical trials early next year.
The data, which has not yet been peer reviewed, tested blood from people who had received the vaccine against a pseudovirus engineered to resemble the variant. It is similar to data discussed by the top US infectious disease expert, Dr Anthony Fauci.
Burton said it would be for governments and regulators to gauge whether they wanted the enhanced level of protection that a 100µg dose might confer.
The company said the 100µg dose was generally safe and well tolerated, although there was a trend toward slightly more frequent adverse reactions.
Omicron, a highly contagious variant first detected last month in southern Africa and Hong Kong, has raced around the world and been reported in 89 countries, according to the World Health Organization.
It said the number of Omicron cases was doubling in one and a half to three days in areas with community transmission, but noted that much remained unknown about the variant, including the severity of the illness it causes.
- by foxnews
- descember 09, 2016
Ancient Ten Commandments fragment of 2,000-year-old manuscript to go on display at Reagan Library
The "Dead Sea Scrolls" exhibit, announced at the Ronald Reagan Presidential Library and Museum, features ancient Jewish manuscripts, plus the rarely seen Ten Commandments Scroll.
read more


